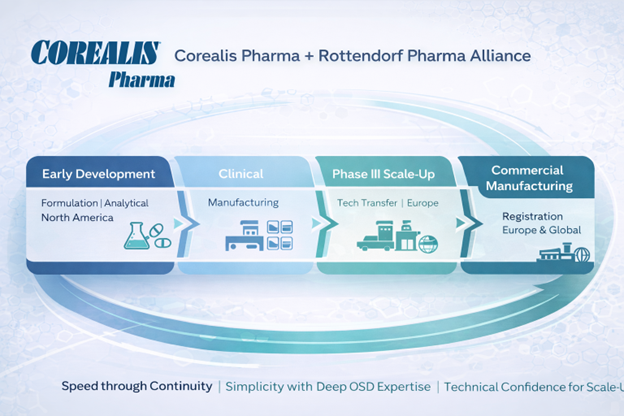
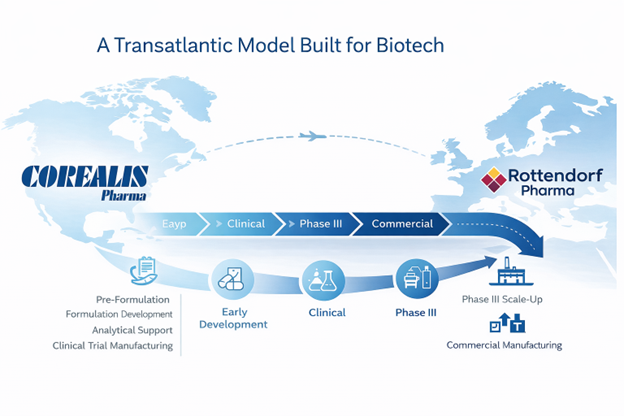
Corealis Pharma Inc. is a privately held, GMP-compliant CDMO based in Laval, Québec, Canada. Specializing in oral solid dosage forms, Corealis provides customized formulation development, analytical, and manufacturing services for biopharmaceutical companies worldwide. Known for its flexibility, scientific expertise, and quality-driven approach, Corealis is a trusted partner in the development of complex drug products. Learn more at www.corealispharma.com.
Rottendorf Pharma is one of the leading contract manufacturers and developers in the field of solid oral dosage forms. As a CDMO (Contract Development and Manufacturing Organization), Rottendorf Pharma is active in the manufacture and packaging as well as
the development of formulations and analytical processes for the international pharmaceutical industry. The company manufactures a wide range of original drugs, producing more than 5 billion tablets annually. Its customers include large, small, virtual, and global pharmaceutical and biotechnology companies. Rottendorf Pharma does not market its own products and therefore never competes with its own customers. Rottendorf Pharma employs more than 1,400 people. The company was founded in Berlin in 1928 by Andreas Rottendorf. Since 1949, the headquarters for production and administration has been located in Ennigerloh in the Münsterland region, the home of the company’s founder. The company has been foundation-managed since 1974, with the Rottendorf Foundation supporting charitable causes. Rottendorf Pharma has been represented in the USA since 2011.