Bora Pharmaceuticals and Corealis Pharma Forge Strategic Alliance to Deliver Seamless, End-to End Oral Solid Dose Development
Partnership creates simplified pathway from early development commercial launch
Partnership creates simplified pathway from early development commercial launch

One Alliance. No Surprises.
Empowering biotech and pharmaceutical innovators with a faster, simpler, and a more scalable path from development to commercialization.
|
One Alliance |
No Surprises |
 |
 |
| Access the value of an integrated team that moves as one
· SMEs unite R&D, formulation, tech transfer, scale-up, Phase III, and commercialization expertise to accelerate your journey · Unified steering committee and shared quality framework ensure every step is aligned from day one · With unified proposals, coordinated business development, and harmonized equipment and process documentation, we surround every program with a seamless ecosystem built for speed, quality, and confidence. |
No guesswork. No confusion. Just a clear, streamlined path from early development to commercial launch
· Coordinated execution and shared timelines make reliability a guarantee · Joint delivery team keeps your program on track and on schedule, ensuring every milestone is hit on time. |
Unified steering committee, shared quality framework, joint delivery team and timelines from Day One
Bora and Corealis operate as a single integrated team—uniting R&D, formulation, tech transfer, scale-up, Phase III, and commercial manufacturing. With one steering committee, one quality framework, and one coordinated delivery team, your program moves faster with fewer hand-offs and zero surprises.

Drug development is complex. Your path shouldn’t be.
An integrated model designed for speed and clarity.
Result: A clear, coordinated route to market—without any friction.

• Single proposal, single point of contact
• Seamless tech transfer
• Consistent quality across sites
• Aligned timelines and proactive communication
No confusion. No guesswork. Just a streamlined, reliable OSD journey.
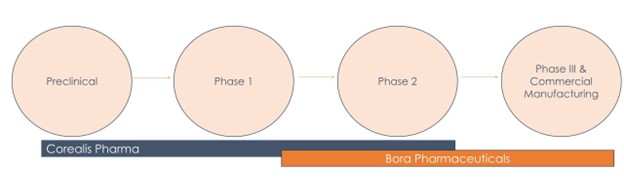
The partnership between Bora and Corealis exemplifies a transformative approach in the biotech and pharmaceutical landscape. By integrating our expertise, we offer a streamlined, efficient pathway that minimizes uncertainties, ensuring that innovators can focus on their core objectives with confidence. This collaboration not only enhances operational efficiency but also fosters innovation, enabling a quicker transition from concept to market-ready products.
Corealis Pharma Inc. is a privately held, GMP-compliant CDMO based in Laval, Québec, Canada. Specializing in oral solid dosage forms, Corealis provides customized formulation development, analytical, and manufacturing services for biopharmaceutical companies worldwide. Known for its flexibility, scientific expertise, and quality-driven approach, Corealis is a trusted partner in the development of complex drug products. Learn more at www.corealispharma.com.
Bora Pharmaceuticals is a premier international contract development and manufacturing organization (CDMO) specializing in formulation development, clinical and commercial manufacturing, and packaging of complex oral solid dose, liquid, semi-solid, biologic, and sterile injectable pharmaceutical products. From its world-class sites in North America and Asia, Bora delivers drug products with unparalleled quality to more than 100 markets around the world. Bora Pharmaceuticals Co., Ltd. is publicly traded on the Taiwan Stock Exchange (TWSE: 6472). For more information, visit www.boracdmo.com.
Ready to simplify your OSD pathway?
Corealis’ experts are ready to answer any queries about the company’s oral solid dose offering, including its development and manufacturing capabilities.
